|
Getting your Trinity Audio player ready... |
The f₂ Similarity Factor in Dissolution Testing-Finished Products
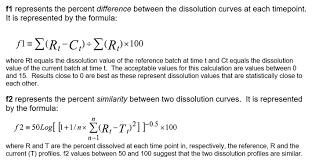
f₂ Similarity Factor: One of the most common questions that arises during discussions on dissolution testing is: What exactly is the f₂ factor, and how should it be applied in practice? The f₂ factor, often called the similarity factor, is a mathematical tool that evaluates how closely two dissolution profiles resemble one another. Among several statistical measures available, the f₂ is by far the most widely used in pharmaceutical development and regulatory assessments.
In simple terms, the f₂ value provides a quantitative way of comparing two sets of dissolution data. Most frequently, it is used to demonstrate equivalence between a generic drug and its reference or innovator product. However, its applications are not limited to generic–innovator comparisons. Researchers and manufacturers also use it in other contexts, such as when modifying formulations, making manufacturing changes, or evaluating alternative dissolution conditions.
The Basis of the f₂ Calculation: f₂ Similarity Factor
The f₂ factor is derived by comparing two dissolution profiles across multiple time points. According to regulatory recommendations, at least three time points are required for a valid comparison, though more points are usually included to properly capture the shape of the dissolution curve.
To ensure meaningful and reliable results, certain conditions must be satisfied before applying the f₂ calculation:
- Variability requirements:
- At the earliest time point, the percentage coefficient of variation (%CV) should not exceed 20%.
- For all subsequent time points, variability should be less than 10%.
- Updated regulatory guidelines also specify that within the first 10 minutes of testing, variability should remain under 20%, while for all later time points it must be within 10%.
- Selection of time points:
The chosen sampling times must adequately represent the dissolution process. Importantly, no more than one time point should show a dissolution value greater than 85% for either product being compared. This rule prevents bias from overly rapid dissolution, which could make the two profiles appear more similar than they actually are.
- Sample size:
Typically, 12 dosage units (n = 12) are used for each formulation in the comparison. This ensures statistical robustness and compliance with regulatory expectations.
The output of the f₂ calculation is a value between 0 and 100. A result of 50 or greater indicates that the two dissolution curves are similar. A perfect match between curves would theoretically yield a value of 100. In practice, values slightly above 50 are usually sufficient to support similarity, while values far below 50 suggest significant differences between the profiles.
Practical Applications of the f₂ Factor: f₂ Similarity Factor
- Generic vs. Innovator Comparisons
The most recognized application of the f₂ factor is in the demonstration of bioequivalence between a proposed generic drug and its reference listed drug (RLD). Regulatory authorities such as the FDA and EMA often accept f₂ values ≥ 50 as evidence that the dissolution characteristics of a generic closely match those of the innovator product. Since dissolution is considered a critical quality attribute that impacts drug absorption, showing similarity supports the case for therapeutic equivalence.
- Formulation Modifications
Beyond generic approval, the f₂ factor plays a key role in post-approval changes. Manufacturers frequently adjust formulations during a product’s lifecycle—for example, by changing excipients, modifying manufacturing processes, or even transferring production to another facility. In such cases, the f₂ test provides a straightforward way to demonstrate that the updated product dissolves in the same manner as the original, thus reassuring regulators and patients that product performance remains consistent.
- Evaluation of Method Changes
Another valuable application of f₂ lies in dissolution method development and validation. Scientists may want to test the impact of modifying certain parameters within the dissolution method, such as:
- Using different sinkers to hold dosage units in place.
- Applying alternative degassing techniques to remove air bubbles.
- Adjusting agitation speeds or apparatus conditions.
By comparing the dissolution profile of a drug before and after a procedural change, the f₂ factor can confirm whether the modification alters product performance significantly. If the profiles remain similar, the change can often be adopted without raising concerns about bioequivalence.
Interpreting the f₂ Factor in Context:f₂ Similarity Factor
f₂ Similarity Factor: While the f₂ is an extremely useful statistical tool, it is not without limitations. The method assumes relatively low variability, and if variability exceeds the recommended thresholds, the calculation may not yield meaningful results. In such cases, alternative statistical approaches—such as multivariate analyses, bootstrap methods, or model-dependent comparisons—may be more appropriate.
Additionally, the f₂ factor is most effective when applied to immediate-release formulations, where dissolution occurs within a relatively short timeframe. For extended-release or controlled-release products, the dissolution process can be more complex, and regulatory agencies often require additional analyses alongside f₂.
Another consideration is that while a value ≥ 50 suggests similarity, the number itself does not convey the degree of therapeutic equivalence. Dissolution testing is an in vitro surrogate for drug release, and while critical, it is just one component of overall bioequivalence assessment. Pharmacokinetic studies in humans often remain necessary to confirm clinical equivalence.
Why the f₂ Factor Matters:f₂ Similarity Factor
The pharmaceutical industry operates under rigorous quality standards, and consistency in drug performance is essential for patient safety and therapeutic success. The f₂ factor provides a simple yet powerful way to ensure that two products—or two versions of the same product—deliver drug release in a comparable manner.
For regulatory bodies, f₂ offers a standardized benchmark that simplifies the review process. For manufacturers, it provides a clear target during product development and lifecycle management. And for patients, it ultimately translates into confidence that generic drugs and reformulated products will perform just as reliably as their brand-name counterparts.
Conclusion
The f₂ similarity factor remains one of the most important and widely accepted tools in dissolution testing. By mathematically comparing dissolution profiles, it helps determine whether two drug products can be considered similar in terms of in vitro release characteristics. Whether applied in generic drug development, formulation adjustments, or method validation, f₂ supports critical decisions that impact both regulatory approval and patient safety.
Although it has its limitations, especially in cases of high variability or complex dosage forms, the f₂ continues to serve as a practical, reliable, and efficient method for establishing dissolution profile similarity. As pharmaceutical science evolves, complementary tools may be used alongside f₂, but its role as a cornerstone of dissolution testing is unlikely to diminish.
In short, the f₂ factor is more than just a number—it is a bridge that links laboratory dissolution data to real-world confidence in drug performance.