(COPD) Updated Guidelines for Managing Chronic Obstructive Pulmonary Disease
(COPD) Updated Guidelines for Managing Chronic Obstructive Pulmonary Disease COPD: Chronic Obstructive Pulmonary Disease refers to a set of progressive…
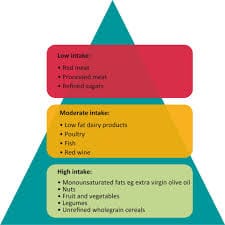
Guidelines for Obesity Management-2025
Guidelines for Obesity Management Obesity is a chronic medical condition that significantly impacts overall health. The American Medical Association (AMA)…
Guidelines for Asthma Management (Updated 2023)
Guidelines for Asthma Management (Updated 2023) Asthma is a widespread chronic respiratory condition characterized by reversible airway obstruction, bronchospasm, and…
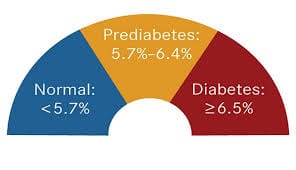
Comprehensive Guidelines for Managing Prediabetes Updated: October 2025
Comprehensive Guidelines for Managing Prediabetes Updated: October 2025 Prediabetes is a metabolic condition where blood glucose levels are elevated beyond…
Validating a SCADA System within the Framework of Computer System Validation (CSV)
Validating a SCADA System within the Framework of Computer System Validation (CSV) SCADA In regulated industries such as pharmaceuticals, biotechnology,…
Understanding the Differences Between Industry 1.0, 2.0, 3.0, and 4.0
Understanding the Differences Between Industry 1.0, 2.0, 3.0, and 4.0 Industry :Over the course of history, the industrial sector has…
Managing Computer System Validation (CSV) in the Pharmaceutical Sector-25
Managing Computer System Validation (CSV) in the Pharmaceutical Sector Computer System Validation: In the pharmaceutical industry, maintaining high-quality standards isn’t…
ERP System Validation in Regulated Industries-v1
ERP System Validation in Regulated Industries ERP System Validation: Validating an Enterprise Resource Planning (ERP) system is a critical step…
Re-Validation in Computerized System Validation (CSV) 2
Re-Validation in Computerized System Validation (CSV) Computerized System Validation (CSV): In the context of Computerized System Validation (CSV), re-validation is…
Objectives of Data Management and Integrity in the Pharmaceutical Industry
Objectives of Data Management and Integrity in the Pharmaceutical Industry Data Management: In today’s pharmaceutical industry, data serves as the…