|
Getting your Trinity Audio player ready... |
Pharmaceutical Calculations | Moles And Molarity – Part 2 (Expanded Version)
Understanding Molarity and Its Importance
Molarity : In a pharmaceutical or laboratory setting, the concentration of solutions plays a critical role in ensuring the accurate preparation and use of chemical substances. One of the most common ways to express the concentration of a solution is through molarity. Molarity provides an essential standard for understanding the concentration of a solute in a solution.
What is Molarity?
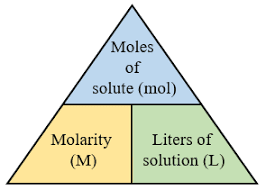
Molarity, often denoted by the symbol MMM or in units of mol/L (moles per liter), is defined as the number of moles of solute dissolved in one liter of solution. It is a key measure used to quantify the concentration of a solution. Molarity is commonly expressed as:
This measure tells you how many moles of a substance are present in one liter of solution. For instance, if you have a 1 M solution of NaCl (sodium chloride) in water, it means there is exactly 1 mole of NaCl dissolved in 1 liter of water. Similarly, for a solution of oxalic acid in acetone with a molarity of 0.05 M, it means there are 0.05 moles of oxalic acid per 1 liter of acetone.
Common Molarity Examples
- 1 M of NaCl in water means 1 mole of NaCl is dissolved in 1 liter of water.
- 0.05 M of oxalic acid in acetone means 0.05 moles of oxalic acid are dissolved in 1 liter of acetone.
- 0.03 M of aspirin in ethanol indicates 0.03 moles of aspirin are dissolved in 1 liter of ethanol.
Laboratory Applications of Molarity
In laboratory work, particularly in fields like pharmaceutical sciences, chemists often need to prepare solutions with specific molar concentrations. The precision and consistency of these solutions are critical for experiments, reactions, or formulations. Molarity simplifies these processes by allowing easy calculations of solute amounts needed for a particular volume of solution.
It is essential to note that in real-world applications, the concentration is frequently adjusted to achieve optimal effects, whether it’s for a reaction, dosing medication, or conducting analytical tests.
Molarity Calculation Formula
Molarity calculations rely on a basic relationship between the number of moles of solute, the volume of the solution, and the molarity itself. The formula for molarity is given as:
Where:
- MMM is the molarity (in mol/L),
- nnn is the number of moles of solute (in mol),
- VVV is the volume of the solution (in L).
Example Problems and Solutions
Problem 1: Molarity Calculation from Moles and Volume
Problem:
You have 0.02 moles of D-Xylose dissolved in 160 mL of water. What is the molarity of the solution?
Solution:
Step 1:
Identify the formula to use. The formula for molarity is:
Where nnn is the number of moles and VVV is the volume of the solution in liters.
Step 2:
Convert the volume from milliliters to liters:
160mL-0.160L
Step 3:
Now substitute the given values into the formula:
Answer:
The molarity of the solution is 0.125 mol/L or 0.125 M.
Problem 2: Determining Mass of Solute from Molarity and Volume
Problem:
An unknown amount of the antifungal miconazole (C18_{18}18H14_{14}14Cl4_{4}4N2_{2}2O) is dissolved in 50 mL of solvent to form a solution with a concentration of 0.001 M. How much miconazole (in grams) was used?
Solution:
Step 1:
Start by identifying the relevant formula. We are given molarity and volume, and we need to find the mass of the solute. First, use the molarity formula to find the moles of solute:
n=M×V
Where:
- M-0.001 mol/L(molarity),
- V-0.050 L (volume in liters).
Step 2:
Calculate the number of moles of miconazole:
n=0.001mol/L×0.050L=0.00005mol
Step 3:
Convert moles to mass using the molecular weight of miconazole. The molecular weight of miconazole is 416.13 g/mol. Now, calculate the mass:
Mass=n× Molecular Weight=0.00005mol×416.13g/mol=0.0208g
Step 4:
Convert grams to milligrams:
0.0208 g-20.8 mg
Answer:
The mass of miconazole used to prepare the solution is 20.8 mg.
Problem 3: Molarity Calculation for a Solid Solute
Problem:
A trainee prepared a solution of cis-(−)-carveol (molecular weight 152.24 g/mol) by dissolving 2200 mg of cis-(−)-carveol in 20 mL of solvent. What is the molarity of this solution?
Solution:
Step 1:
First, convert the mass of cis-(−)-carveol from milligrams to grams:
2200mg-2.2g
Step 2:
Next, calculate the number of moles of cis-(−)-carveol using its molecular weight:
Step 3:
Now, convert the volume of solvent to liters:
20mL-0.020L
Step 4:
Finally, calculate the molarity using the formula:
Answer:
The molarity of the solution is 0.72 M.
Problem 4: Preparing a Solution from Molarity and Volume
Problem:
You are tasked with preparing 50 mL of a 25 mM aqueous solution of KBr. How much KBr (in grams) do you need to dissolve in the solvent?
Solution:
Step 1:
Convert the volume from milliliters to liters:
50mL-0.050L
Step 2:
Convert the concentration from millimolar (mM) to molar (M):
25mM-0.025M
Step 3:
Calculate the number of moles of KBr using the molarity and volume:
Step 4:
Calculate the mass of KBr using its molecular weight. The molecular weight of KBr is the sum of the atomic weights of K (39.1 g/mol) and Br (79.9 g/mol), which equals:
Molecular Weight of KBr=39.1g/mol+79.9g/mol=119.0g/mol
Now, calculate the mass:
Mass=n×Molecular Weight=0.00125mol×119.0g/mol=0.14875g
Answer:
You will need 0.14875 g of KBr to prepare the 50 mL 25 mM aqueous solution.
Summary Table for Molarity Calculations
| Problem | Given | Formula | Solution |
| 1 | 0.02 mol of D-Xylose in 160 mL water | 0.125 mol/L0.125 \, \text{mol/L}0.125mol/L | |
| 2 | 50 mL solvent, 0.001 M Miconazole | 20.8 mg | |
| 3 | 2200 mg cis-(−)-carveol in 20 mL solvent | 0.72 M | |
| 4 | 50 mL of 25 mM KBr | 0.14875 g |
Conclusion
Molarity is a powerful concept in laboratory chemistry, especially in the preparation of pharmaceutical solutions. The ability to calculate molarity and understand how to use it for different substances and volumes is essential for researchers and healthcare professionals. By following these systematic steps, you can ensure precise and reliable solution preparations for experiments or formulations.