|
Getting your Trinity Audio player ready... |
High Performance Liquid Chromatography (HPLC): An In-Depth Overview
High Performance Liquid Chromatography (HPLC) is a vital analytical technique used to separate, identify, and quantify components within a mixture. As one of the most advanced and efficient forms of chromatography, HPLC plays a crucial role in various industries such as pharmaceuticals, food safety, clinical research, environmental monitoring, and forensic analysis. This article explores the fundamental principles of HPLC, its mechanisms, components, applications, and the different types of detectors commonly used in this technology.
1. Principle of HPLC: High Performance Liquid Chromatography
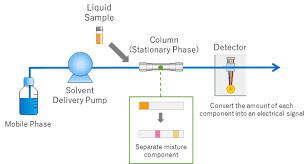
HPLC operates on the principle of liquid chromatography, a technique based on the differential interaction of various components of a sample with the stationary and mobile phases. In simple terms, the sample is injected into a chromatographic column, where it interacts with the stationary phase and is carried through by the mobile phase. The different components of the sample move at varying rates due to differences in their affinity for the stationary phase, leading to separation.
The sample is then detected as it exits the column, often in the form of a chromatogram, which represents the detector’s signal as a function of time or elution volume. The time it takes for a component to pass through the column and reach the detector is called the retention time (t_R), which is characteristic of that specific compound under certain conditions.
2. The Scope and Applications of HPLC
HPLC has vast applications in various fields, primarily in sectors where purity and precise quantification of substances are paramount. Below is a breakdown of HPLC’s application scope across different industries:
| Field | Typical Mixtures |
| Pharmaceuticals | Antibiotics, sedatives, steroids, analgesics, crude drugs, cosmetics |
| Biochemical | Amino acids, proteins, peptides, carbohydrates, lipids, enzymes, medicines, hormones |
| Food Products | Mycotoxins, additives, saccharides, amino acids, vitamins, fatty acids, coloring agents, antibacterials |
| Industrial Chemicals | Condensed aromatics, surfactants, propellants, dyes, polymers, plasticizers |
| Forensic Chemistry | Drugs, poisons, blood alcohol, narcotics |
| Environmental Field | Inorganic ions, organic acids, agricultural chemicals, pesticides, herbicides, phenols |
| Clinical Medicine | Bile acids, drug metabolites, urine extracts, estrogens |
As evident from this table, HPLC’s versatility allows it to handle a wide range of substances, ensuring its broad applicability in real-world analysis.
3. Instrumentation in HPLC
The essential components of an HPLC system include the pump, injector, column, detector, and data system. Below is a detailed breakdown of each:
- Pump: The pump is responsible for delivering the mobile phase under controlled pressure. It ensures that the solvent and sample pass through the column at the appropriate flow rate.
- Injector: The injector is the device used to introduce the sample into the mobile phase. It precisely injects a small, defined volume of the sample into the system.
- Column: The column is the heart of the chromatographic system. It contains the stationary phase, which is typically a silica-based material. The column is where separation of compounds occurs based on their interactions with the stationary phase.
- Detector: The detector records the components as they elute from the column. Various types of detectors, such as UV/Vis detectors, fluorescence detectors, and mass spectrometers, can be used depending on the application.
- Data System: The data system collects and processes the signal from the detector, generating a chromatogram that represents the analysis results.
4. Separation Mechanisms in HPLC
The principle behind the separation of components in HPLC lies in the differences in their interactions with the stationary phase. These interactions cause different components to travel through the column at different rates, thereby separating them.
- Stationary Phase: The stationary phase is typically a solid material, such as silica gel, packed into a column. The stationary phase interacts with the sample, and this interaction varies for different compounds.
- Mobile Phase: The mobile phase is a liquid solvent or a mixture of solvents that moves through the column, carrying the sample components with it. The type of mobile phase used can significantly affect the separation process.
5. Types of Chromatographic Separation Modes
HPLC can be performed using various separation modes, each suitable for specific types of analytes. The primary separation modes include:
- Normal Phase Chromatography (NPC):
- Stationary Phase: Highly polar (hydrophilic), such as silica gel.
- Mobile Phase: Low polarity (hydrophobic), like non-polar solvents (hexane).
- Reversed Phase Chromatography (RPC):
- Stationary Phase: Low polarity (hydrophobic), such as octadecylsilane (C18) bonded to silica.
- Mobile Phase: High polarity (hydrophilic), like water mixed with organic solvents (methanol, acetonitrile).
- Ion Chromatography (IC):
- This mode separates ions and polar molecules based on their charge.
- Size Exclusion Chromatography (SEC):
- In SEC, the separation is based on the size of the molecules, typically used for large biomolecules like proteins.
- Affinity Chromatography (AC):
- This mode uses the specific interaction between an analyte and a ligand to achieve separation.
6. Common Detectors in HPLC
HPLC offers a variety of detectors, each suited to different types of analytes. Below is an overview of the most commonly used detectors and their characteristics:
| Detector Type | Applications | Advantages | Disadvantages |
| UV/Vis Detector | Compounds with UV or visible absorption, such as aromatic compounds and double bonds | High sensitivity, robust to changes in temperature and flow rate, compatible with gradient elution | Only detects compounds that absorb UV/Vis light |
| PDA Detector | Compounds with UV/Vis spectra, for compound identification and peak purity analysis | Can analyze a sample at multiple wavelengths, stores UV spectra for compound identification | Slightly less sensitive than UV/Vis detector |
| Fluorescence Detector | Fluorescent compounds, such as polycyclic aromatic hydrocarbons and certain proteins | Higher sensitivity than UV/Vis, selective detection, good for trace analysis | Not all compounds are fluorescent, and sensitive to environmental factors like pH and temperature |
| Conductivity Detector | Ionic compounds, ideal for ion chromatography, such as inorganic ions or acids | High sensitivity for low-concentration ionic species, suitable for environmental and food analysis | Not compatible with gradient elution, sensitive to fluctuations in mobile phase composition |
| Refractive Index Detector | Non-UV-active compounds, such as sugars, polymers, and lipids | Universal detection for nearly all solutes, not affected by flow rate | Less sensitive, incompatible with gradient elution |
| Mass Spectrometer (MS) | Compounds that can be ionized, for detailed structure identification | Highly sensitive, provides molecular weight and structural information | Requires complex instrumentation, not as simple as other detectors |
Each detector type has its pros and cons, and the selection depends on the type of sample and the required sensitivity and selectivity.
7. Isocratic vs. Gradient Elution in HPLC
- Isocratic Elution: In isocratic elution, the composition of the mobile phase remains constant throughout the chromatographic run. This technique is simple to use and ideal for analyzing compounds that elute at similar rates.
- Gradient Elution: In gradient elution, the composition of the mobile phase gradually changes during the analysis. This technique is useful for separating compounds with a wide range of polarities or when complex mixtures are involved.
Both methods are important in HPLC, and the choice depends on the nature of the sample being analyzed.
8. Guard Columns and Pre-Columns in HPLC
To ensure the longevity and efficiency of the main analytical column, guard columns and pre-columns are often used:
- Guard Column: A guard column is designed to protect the main column from contaminants or particulate matter present in the sample. It traps impurities before they reach the analytical column.
- Pre-Column: A pre-column is typically used to remove impurities in the mobile phase, ensuring that the sample is clean before injection into the column.
Conclusion
HPLC remains one of the most powerful and versatile analytical techniques available for the separation, identification, and quantification of components in complex mixtures. From pharmaceuticals to environmental science, its applications are widespread, ensuring the safety, efficacy, and quality of products across multiple sectors.
By understanding the various mechanisms, instrumentation, detectors, and separation modes involved, professionals can optimize their HPLC systems for a wide range of analyses, enabling high-resolution and highly sensitive results