|
Getting your Trinity Audio player ready... |
Dissolution f₂ in Multimedia a is True Indicator of Bioequivalence?
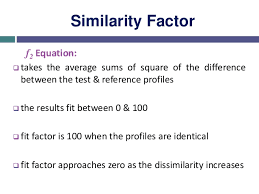
Dissolution f₂ in pharmaceutical sciences, ensuring that a generic product behaves in the same way as its reference drug is central to regulatory approval and therapeutic confidence. One of the mathematical tools frequently applied for this purpose is the similarity factor, widely known as f₂. This parameter compares the dissolution profiles of a test product against its reference listed drug (RLD) and provides a quantitative indication of how similar the two profiles are under specific testing conditions.
The principle is straightforward: when the calculated f₂ value is 50 or greater, it is generally accepted that the two dissolution profiles are similar. Regulatory bodies such as the United States Food and Drug Administration (USFDA), the European Medicines Agency (EMA), and the World Health Organization (WHO) recognize this threshold as valid. However, they also emphasize that this conclusion is only reliable within defined experimental limits. Typically, it is used in a single dissolution medium under controlled laboratory conditions.
This brings us to an important and debated question: if f₂ is applied across multiple dissolution media, does it serve as a genuine indicator of bioequivalence (BE)? To answer this, we must look into the purpose, applications, and limitations of f₂ testing in multimedia environments.
The Purpose of f₂ in Multimedia Dissolution Testing Dissolution f₂
The gastrointestinal (GI) tract is a highly dynamic system, where the pH gradually shifts from acidic in the stomach (around pH 1–2) to more neutral and alkaline conditions in the small intestine (pH 4.5–6.8 and beyond). Since most orally administered drugs pass through these environments before absorption, it is essential to evaluate how a formulation behaves under such varying conditions.
When dissolution tests are conducted in multiple pH media, usually at pH 1.2, 4.5, and 6.8, they aim to mimic the physiological environment encountered by the dosage form. If a formulation exhibits similar dissolution behavior in all these media, reflected by consistent f₂ values, it suggests that the drug release is robust and pH-independent. This strengthens confidence in the stability and reproducibility of the formulation.
However, this type of evaluation is still in vitro—a laboratory simulation of drug release. While it provides meaningful scientific data about how a dosage form reacts to environmental changes, it does not capture the full complexity of what happens once the drug enters the human body.
The Limitations of f₂ as a Surrogate for Bioequivalence Dissolution f₂
Although the f₂ factor across multiple dissolution media is valuable, it cannot be viewed as a standalone measure of bioequivalence. Bioequivalence is a clinical concept, defined as the absence of a significant difference in the rate and extent of drug absorption between the test product and the reference drug.
Dissolution tests only capture how quickly and completely a drug is released from its dosage form into solution. They do not measure absorption into the bloodstream, distribution to tissues, metabolism, or excretion. These latter steps are influenced by a variety of physiological factors, including:
Gastrointestinal motility: The speed at which the stomach empties and intestinal transit time can greatly affect drug absorption.
Enzymatic activity: Enzymes present in the GI tract or liver may degrade drugs before they reach systemic circulation.
Permeability: Even if dissolution is similar, drugs with low permeability (e.g., BCS Class III or IV compounds) may not achieve comparable plasma levels.
Food effects: The presence of food alters gastric emptying, bile secretion, and drug solubility.
First-pass metabolism: Drugs may undergo significant metabolism before reaching systemic circulation, which dissolution testing cannot predict.
Because of these complexities, an identical f₂ value in dissolution studies does not guarantee equivalent in vivo exposure. This limitation is particularly significant for Biopharmaceutics Classification System (BCS) Class II and IV drugs, where either solubility or permeability is the rate-limiting step for absorption. For such drugs, f₂ similarity in vitro cannot reliably predict clinical performance.
When Can f₂ Be Supportive in Bioequivalence Assessments? Dissolution f₂
Despite its limitations, the f₂ factor has clear value in both regulatory submissions and formulation development. Regulatory agencies accept f₂ analysis as supportive evidence in specific contexts:
Post-approval changes
When a manufacturer changes manufacturing sites, excipient suppliers, or minor formulation aspects, regulators often request comparative dissolution testing. Demonstrating similarity through f₂ can help ensure that such changes do not affect product performance.
Site and scale transfers
When production is scaled up or transferred to a different facility, f₂ analysis provides reassurance that the new batches behave consistently with those previously approved.
Formulation modifications
Minor adjustments, such as changing a coating polymer or modifying tablet hardness, can be assessed through f₂ without immediately requiring new clinical studies.
Biowaivers for BCS Class I and III drugs
For drugs classified as highly soluble (Class I) or highly soluble but low permeability (Class III), regulatory agencies may grant a waiver of in vivo BE studies if certain strict conditions are met. Among these conditions is the demonstration of dissolution profile similarity across multiple media using f₂. However, agencies still require a comprehensive justification package, including stability data, excipient compatibility, and risk assessments.
Therefore, while f₂ cannot serve as a direct replacement for bioequivalence studies, it can support regulatory decision-making and facilitate drug development when used in the right context.
Broader Scientific Perspective on f₂ Testing: Dissolution f₂
It is important to note that f₂ analysis itself has methodological boundaries. For instance:
f₂ is most reliable when dissolution results are not overly variable. If variability is high, the similarity factor may not be valid.
It is generally applied when the number of dissolution time points is between 3 and 15, with at least 12 units tested per batch.
The metric is more meaningful when at least one time point shows incomplete dissolution (below 85%), since complete dissolution at early time points renders comparison less informative.
Researchers have also highlighted that f₂ can sometimes mask subtle but clinically relevant differences. For example, two profiles might be judged “similar” based on the calculation, but one formulation could still exhibit slightly faster or slower initial release, potentially influencing onset of action or therapeutic outcomes. Because of this, some experts advocate for complementary statistical or modeling-based approaches, such as multivariate analysis or dissolution modeling using deconvolution techniques.
Conclusion
The similarity factor f₂, when applied across multiple dissolution media, is a useful but limited tool. It offers valuable insights into the robustness of a formulation and its ability to maintain consistent release across the physiological pH range. This makes it an important element of quality control, regulatory submissions, and development strategies, particularly for post-approval changes or BCS-based biowaivers.
However, f₂ in multimedia should never be mistaken for a definitive measure of bioequivalence. True bioequivalence requires an in vivo demonstration that the rate and extent of absorption are not significantly different between the test and reference product. Since human physiology introduces variables far beyond what can be captured in a dissolution study—such as permeability, metabolism, and food effects—clinical BE studies remain the gold standard unless specific regulatory conditions for a waiver are fulfilled.
In summary, f₂ across multimedia environments should be regarded as supportive evidence, not as a substitute for in vivo bioequivalence testing. It strengthens the scientific foundation of formulation robustness but does not eliminate the need for clinical confirmation in most cases. For regulators, developers, and clinicians alike, this perspective ensures both scientific rigor and patient safety in the approval and use of generic medicines.