Microbiological Data Integrity Issues and Their Control Strategies Microbiological Data Integrity: Microbiology laboratories are fundamentally different from chemistry labs. While…
Read More

Microbiological Data Integrity Issues and Their Control Strategies Microbiological Data Integrity: Microbiology laboratories are fundamentally different from chemistry labs. While…
Read More
Data Integrity in the Pharmaceutical Industry Data Integrity: In the pharmaceutical sector, data integrity is not just a regulatory obligation…
Read More
Data Integrity in Analytical Laboratories Data integrity has become one of the most scrutinized areas in pharmaceutical and analytical laboratories.…
Read More
How Expiry Dates Are Determined for Medicines Expiry Dates When you look at the label of any medicine, you’ll notice…
Read More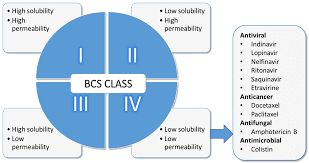
Impact of Granule Particle Size on Dissolution of BCS Class II and IV Drugs Granule Particle Size: Drug development and…
Read More
Static Charge in Pharmaceutical Blending: Origins, Consequences, and Control Strategies Static Charge in Pharmaceutical Blending When scientists and formulators think…
Read More
Forced Degradation Studies for API Selection Forced Degradation Studies Selecting a suitable active pharmaceutical ingredient (API) is a critical step…
Read More
Pharmaceutical Formulation Development Formulation development refers to the process of designing, refining, and producing drug formulations suitable for commercial use.…
Read More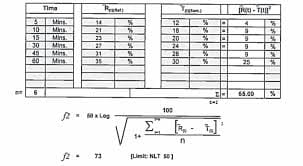
Online f1 and f2 factor calculator in dissolution software free Online f1 and f2 factor: A biowaiver refers to the…
Read More
Computer System Validation in the Pharmaceutical Industry Computer System Validation (CSV) is a structured process applied to computerized systems used…
Read More